How to Solve for Density – Density refers to the measurement of the amount of mass of a substance per unit of volume. This measurement of a pure substance has the same value as its mass concentration. Densities vary with different materials or substances. Moreover, this particular measurement of a material can be relevant to purity, buoyancy, and packaging.
One can simplify this measurement’s comparisons across the different systems of units. The replacement of this measurement with a concept is known as relative density can sometimes take place. Relative densities refer to the ratio of a substance’s density to a standard substance or material, usually water.
Table of Contents
The densities of materials show variation with pressure and temperature. This variety is comparatively small for solids and liquids but significantly greater when it comes to gases. When the pressure on an object increases, then consequently the volume decreases. This ultimately causes an increase in this particular measurement of the object.
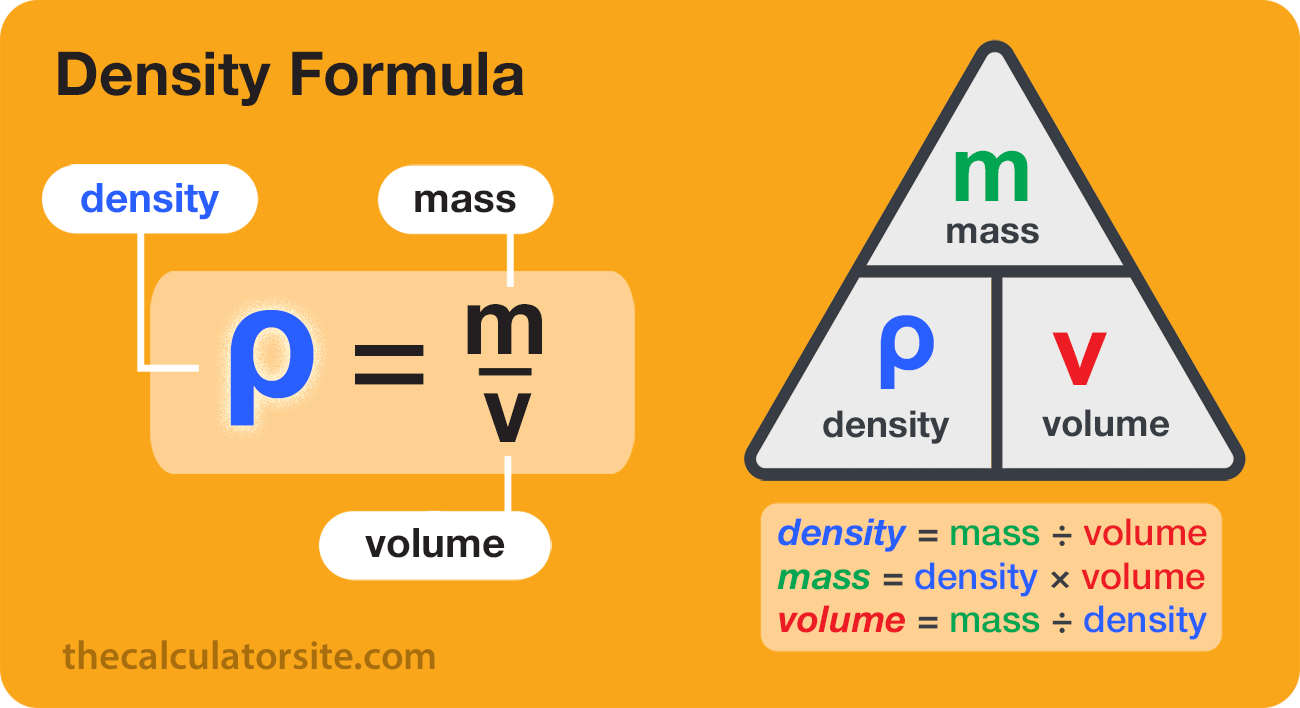
density, mass of a unit volume of a material substance. The formula for density is d = M/V, where d is density, M is mass, and V is volume. Density is commonly expressed in units of grams per cubic centimetre. For example, the density of water is 1 gram per cubic centimetre, and Earth’s density is 5.51 grams per cubic centimetre. Density can also be expressed as kilograms per cubic metre (in metre-kilogram-second or SI units).
For example, the density of air is 1.2 kilograms per cubic metre. The densities of common solids, liquids, and gases are listed in textbooks and handbooks.
Density offers a convenient means of obtaining the mass of a body from its volume or vice versa; the mass is equal to the volume multiplied by the density (M = Vd), while the volume is equal to the mass divided by the density (V = M/d). The weight of a body, which is usually of more practical interest than its mass, can be obtained by multiplying the mass by the acceleration of gravity.
Tables that list the weight per unit volume of substances are also available; this quantity has various titles, such as weight density, specific weight, or unit weight. See also specific gravity. The expression particle density refers to the number of particles per unit volume, not to the density of a single particle, and it is usually expressed as n.
Key Takeaways: How to Calculate Density
- Density is how much matter is contained within a volume. A dense object weighs more than a less dense object that is the same size. An object less dense than water will float on it; one with greater density will sink.
- The density equation is density equals mass per unit volume or D = M / V.
- The key to solving for density is to report the proper mass and volume units. If you are asked to give density in different units from the mass and volume, you will need to convert them.
Question 1: What is the density of a cube of sugar weighing 11.2 grams measuring 2 cm on a side?FEATURED VIDEOhttps://imasdk.googleapis.com/js/core/bridge3.491.0_en.html#goog_10456185700 seconds of 4 minutes, 5 secondsVolume 0% How to Calculate Density
Step 1: Find the mass and volume of the sugar cube.
Mass = 11.2 grams
Volume = cube with 2 cm sides.
Volume of a cube = (length of side)3
Volume = (2 cm)3
Volume = 8 cm3https://66897a815af2dc8cfc3fbd6f3e8dfae8.safeframe.googlesyndication.com/safeframe/1-0-38/html/container.html
Step 2: Plug your variables into the density formula.https://66897a815af2dc8cfc3fbd6f3e8dfae8.safeframe.googlesyndication.com/safeframe/1-0-38/html/container.html
density = mass/volume
density = 11.2 grams/8 cm3
density = 1.4 grams/cm3https://66897a815af2dc8cfc3fbd6f3e8dfae8.safeframe.googlesyndication.com/safeframe/1-0-38/html/container.html
Answer 1: The sugar cube has a density of 1.4 grams/cm3.https://66897a815af2dc8cfc3fbd6f3e8dfae8.safeframe.googlesyndication.com/safeframe/1-0-38/html/container.html
Question 2: A solution of water and salt contains 25 grams of salt in 250 mL of water. What is the density of the salt water? (Use density of water = 1 g/mL)https://66897a815af2dc8cfc3fbd6f3e8dfae8.safeframe.googlesyndication.com/safeframe/1-0-38/html/container.html
Step 1: Find the mass and volume of the salt water.
This time, there are two masses. The mass of the salt and the mass of the water are both needed to find the mass of the salt water. The mass of the salt is given, but the only the volume of water is given. We’ve also been given the density of water, so we can calculate the mass of the water.
densitywater = masswater/volumewater
solve for masswater,
masswater = densitywater·volumewater
masswater = 1 g/mL · 250 mL
masswater = 250 grams
Now we have enough to find the mass of the salt water.
masstotal = masssalt + masswater
masstotal = 25 g + 250 g
masstotal = 275 g
Volume of the salt water is 250 mL.
Step 2: Plug your values into the density formula.
density = mass/volume
density = 275 g/250 mL
density = 1.1 g/mL
Answer 2: The salt water has a density of 1.1 grams/mL.
Finding Volume by Displacement
If you’re given a regular solid object, you can measure its dimensions and calculate its volume. Unfortunately, the volume of few objects in the real world can be measured this easily! Sometimes you need to calculate volume by displacement.
How do you measure displacement? Say you have a metal toy soldier. You can tell it is heavy enough to sink in water, but you can’t use a ruler to measure its dimensions. To measure the toy’s volume, fill a graduated cylinder about half way with water. Record the volume. Add the toy. Make sure to displace any air bubbles that may stick to it. Record the new volume measurement. The volume of the toy soldier is the final volume minus the initial volume. You can measure the mass of the (dry) toy and then calculate density.
Tips for Density Calculations
In some cases, the mass will be given to you. If not, you’ll need to obtain it yourself by weighing the object. When obtaining mass, be aware of how accurate and precise the measurement will be. The same goes for measuring volume. Obviously, you’ll get a more precise measurement using a graduated cylinder than using a beaker, however, you may not need such a close measurement. The significant figures reported in the density calculation are those of your least precise measurement. So, if your mass is 22 kg, reporting a volume measurement to the nearest microliter is unnecessary.
Another important concept to keep in mind is whether your answer makes sense. If an object seems heavy for its size, it should have a high density value. How high? Keep in mind the density of water is about 1 g/cm³. Objects less dense than this float in water, while those that are more dense sink in water. If an object sinks in water, your density value better be greater than 1!
Conclusion
Also, increasing a substance’s temperature results in a decrease in this measurement. This is due to the increase in volume. Heating the bottom of a liquid in most cases results in a decrease in this measurement of such heated liquid.