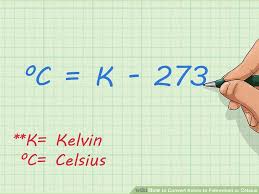There’s a lot of engineering focus on converting kelvin to celsius and vice versa and explaining and discussing and getting into the nitty gritty of why this conversion is meaningful or how it can be useful. But, sometimes we learn best by example. We want to see it in use, we want to understand why we would use it and we want to try it out immediately! Well that’s where this blog comes in. It shows you some real life examples of kelvin to celsius conversions. It gives you some information about the principles involved and then provides you with examples of how you can use them.
Table of Contents
Celsius Scale
Celsius, or centigrade, is a scale and unit of measurement for temperature. It is one of the most commonly used temperature units. Celsius, also known as centigrade, is a scale to measure temperature. The unit of measurement is the degree Celsius (°C). It is one of the most commonly used temperature units in the world. The unit system is named after the Swedish astronomer Anders Celsius (1701-1744), who developed a similar temperature scale.
From 1743 until 1954, 0°C was defined as the freezing point of water, and 100°C was defined as the boiling point of water, both at a pressure of one standard atmosphere, with mercury as the working material. Although these defining correlations are commonly taught in schools today, by international agreement the unit “degree Celsius” and the Celsius scale are currently defined by two different temperatures: absolute zero and the triple point of Vienna Standard Mean Ocean Water (VSMOW; specially purified water). This definition also precisely relates the Celsius scale to the Kelvin scale, which defines the SI base unit of thermodynamic temperature and which uses the symbol K. Absolute zero, the lowest temperature possible (the temperature at which matter reaches minimum entropy), is defined as being precisely 0K and -273.15°C. The temperature of the triple point of water is defined as precisely 273.16K and 0.01°C.
TCelsius = TKelvin − 273.15
Besides expressing specific temperatures along its scale (e.g., “Gallium melts at 29.7646°C” and “The temperature outside is 23 degrees Celsius”), the degree Celsius is also suitable for expressing temperature intervals — differences between temperatures, or their uncertainties (e.g. “The output of the heat exchanger is hotter by 40 degrees Celsius” and “Our standard uncertainty is ±3°C”). Because of this dual usage, one must not rely upon the unit name or its symbol to denote that a quantity is a temperature interval; it must be clear through context or explicit statement that the quantity is an interval.
Kelvin Scale
The kelvin is a unit of measurement for temperature; the null point of the Kelvin scale is absolute zero, the lowest possible temperature. The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units (SI) and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using absolute zero as its null point. In the classical description of thermodynamics, absolute zero is the temperature at which all thermal motion ceases.
The choice of absolute zero as null point for the Kelvin scale is logical. Different types of matter boil or freeze at different temperatures, but at 0K (absolute zero), all thermal motions of any matter are maximally suppressed. The Kelvin scale is used extensively in scientific work because a number of physical quantities, such as the volume of an ideal gas, are directly related to absolute temperature.
The Kelvin scale is named after Glasgow University engineer and physicist William Thomson, 1st Baron Kelvin (1824-1907), who wrote of the need for an “absolute thermometric scale. ” Unlike the degree Fahrenheit and the degree Celsius, the kelvin is not referred to or typeset as a degree. The kelvin is the primary unit of measurement in the physical sciences, but it is often used in conjunction with the degree Celsius, which has the same magnitude. The kelvin is defined as the fraction 1/273.16 of the thermodynamic temperature of the triple point of water (exactly 0.01°C, or 32.018°F). Subtracting 273.16K from the temperature of the triple point of water, 0.01°C, makes absolute zero (0K) equivalent to -273.15°C and -460°F.
Kelvin to Celsius conversion formula
Kelvin and Celsius are two temperature scales. The size of the “degree” for each scale is the same magnitude, but the Kelvin scale starts at absolute zero (the lowest temperature theoretically attainable), while the Celsius scale sets its zero point at the triple point of water (the point at which water can exist in solid, liquid, or gaseous states, or 32.01 F). While the size of the degree is the same between Kelvin and Celsius, there is no point at which the two scales are equal: A Celsius temperature will always be higher than Kelvin. Celsius temperatures can be negative; Kelvin goes down to absolute zero (no negative temperature).
The formula to convert Kelvin into Celsius is C = K – 273.15. All that is needed to convert Kelvin to Celsius is one simple step:
Take your Kelvin temperature and subtract 273.15. Your answer will be in Celsius. The K does not use the word degree or the symbol; depending on the context, generally one or the other (or simply C) is used to report a Celsius temperature.
Example
How many degrees Celsius is 500 K?
C = 500 – 273.15
500 K = 226.85 C
Let’s convert normal body temperature from Kelvin to Celsius. Human body temperature is 310.15 K. Put the value into the equation to solve for degrees Celsius:
C = K – 273.15
C = 310.15 – 273.15
Human body temperature = 37 C
Kelvin to Celsius conversion table
| Kelvin (K) | Celsius (°C) |
|---|---|
| 0 K | -273.15 °C |
| 10 K | -263.15 °C |
| 20 K | -253.15 °C |
| 30 K | -243.15 °C |
| 40 K | -233.15 °C |
| 50 K | -223.15 °C |
| 60 K | -213.15 °C |
| 70 K | -203.15 °C |
| 80 K | -193.15 °C |
| 90 K | -183.15 °C |
| 100 K | -173.15 °C |
| 110 K | -163.15 °C |
| 120 K | -153.15 °C |
| 130 K | -143.15 °C |
| 140 K | -133.15 °C |
| 150 K | -123.15 °C |
| 160 K | -113.15 °C |
| 170 K | -103.15 °C |
| 180 K | -93.15 °C |
| 190 K | -83.15 °C |
| 200 K | -73.15 °C |
| 210 K | -63.15 °C |
| 220 K | -53.15 °C |
| 230 K | -43.15 °C |
| 240 K | -33.15 °C |
| 250 K | -23.15 °C |
| 260 K | -13.15 °C |
| 270 K | -3.15 °C |
| 273.15 K | 0 °C |
| 300 K | 26.85 °C |
| 400 K | 126.85 °C |
| 500 K | 226.85 °C |
| 600 K | 326.85 °C |
| 700 K | 426.85 °C |
| 800 K | 526.85 °C |
| 900 K | 626.85 °C |
| 1000 K | 726.85 °C |
Conclusion
What temperature is an ice cube at? Or the sun? How hot does a steak need to be cooked to kill bacteria? What’s the temperature of boiling water? These are just a few of the questions you can learn to answer by converting Kelvin to Celsius.