Have you ever wondered how you can convert moles to grams? That might seem like a weird question, but it has crossed my mind before. I was reading an article about something scientific and they wanted to know how many grams were in 2 moles of magnesium.
Convert Moles to Grams. How to Convert Grams to Moles? To convert moles of compound X to grams of X, you need to know that one mole is equal to the atomic weight of the molecule in grams. For example, carbon dioxide has a molecular mass of 44 grams. If the problem asks for 150 moles of CO2, then 150 moles X 44 grams/mole = 6300 grams.
Table of Contents
Mole
A mole is the amount of substance of a system, which contains as many elementary entities as there are atoms in 0.012 kilogram (or 12 grams) of carbon-12, where the carbon-12 atoms are unbound, at rest and in their ground state.[1] The number of atoms in 0.012 kilogram of carbon-12 is known as the Avogadro constant, and is determined empirically. The currently accepted value is 6.02214279(30)×1023 mol-1 (2007 CODATA).
According to the SI, the mole is not dimensionless, but has its very own dimension, namely “amount of substance”, comparable to other dimensions such as mass and luminous intensity.[2] (By contrast, the SI specifically defines the radian and the steradian as special names for the dimensionless unit one.)[3] The SI additionally defines the Avogadro constant as having the unit reciprocal mole, as it is the ratio of a dimensionless quantity and a quantity with the unit mole.[3] However, if in the future the kilogram is redefined in terms of a specific number of carbon-12 atoms (see below), then the value of Avogadro’s number will be defined rather than measured, and the mole will cease to be a unit of physical significance.[4]
The relationship of the atomic mass unit (u[5]) to Avogadro’s number means that a mole can also be defined as: That quantity of a substance whose mass in grams is the same as its formula weight. For example, iron has a relative atomic mass of 55.845 u, so a mole of iron has a mass of 55.845 grams. This notation is very commonly used by chemists and physicists.
Scientists and engineers (chemical engineers in particular) sometimes measure amount of substance in units of gram-moles, kilogram-moles, pound-moles, or ounce-moles; these measure the quantity of a substance whose mass in grams, kilograms, pounds, or ounces (respectively) is equal to its formula weight. The SI mole is identical to the gram-mole.
Gram
A gram(g) is a metric unit of mass and was formally defined as the absolute weight of a volume of pure water equal to the cube of the hundredth part of a meter, and at the temperature of melting ice.
While a mole is the unit of measurement for amount of substance in the International System of Units.
A gram is a unit of mass in the metric system defined as one thousandth (1 x 10-3) of a kilogram. Originally, the gram was defined as a unit equal to the mass of one cubic centimeter of pure water at 4°C (the temperature at which water has maximum density). The definition was changed when the base units for the International System of Units (SI) were redefined by the 26th General Conference of Weights and Measures. The change went into effect May 20, 2019.
The symbol for the gram is the lowercase letter “g.” Incorrect symbols include “gr” (the symbol for grains), “Gm” (the symbol for the gigameter), and “gm” (easily confused with the symbol for the gram-meter, g⋅m).https://1553b27a2497c2a80cc364aa56c10e58.safeframe.googlesyndication.com/safeframe/1-0-38/html/container.html
Gram may also be spelled gramme.
Key Takeaways: Gram Definition
- The gram is a unit of mass.
- One gram is one thousandth the mass of one kilogram. The previous definition of the gram was the absolute weight of a 1-centimeter cube of pure water at 4 °C.
- The symbol for the gram is g.
- The gram is a small unit of mass. It is approximately the mass of one small paper clip.
Examples of Gram Weight
Because a gram is a small unit of weight, its size may be difficult for many people to visualize. Here are common examples of objects that have about one gram of mass:
- A small paperclip
- A thumbtack
- A piece of chewing gum
- One US bill
- A pen cap
- One cubic centimeter (milliliter) of water
- A quarter teaspoon of sugar
Uses of the Gram
The gram is widely used in science, particular chemistry and physics. Outside of the United States, the gram is used to measure non-liquid cooking ingredients and produce (e.g., flour, sugar, bananas). Relative composition for food nutrition labels is stated per 100 grams of product, even within the United States
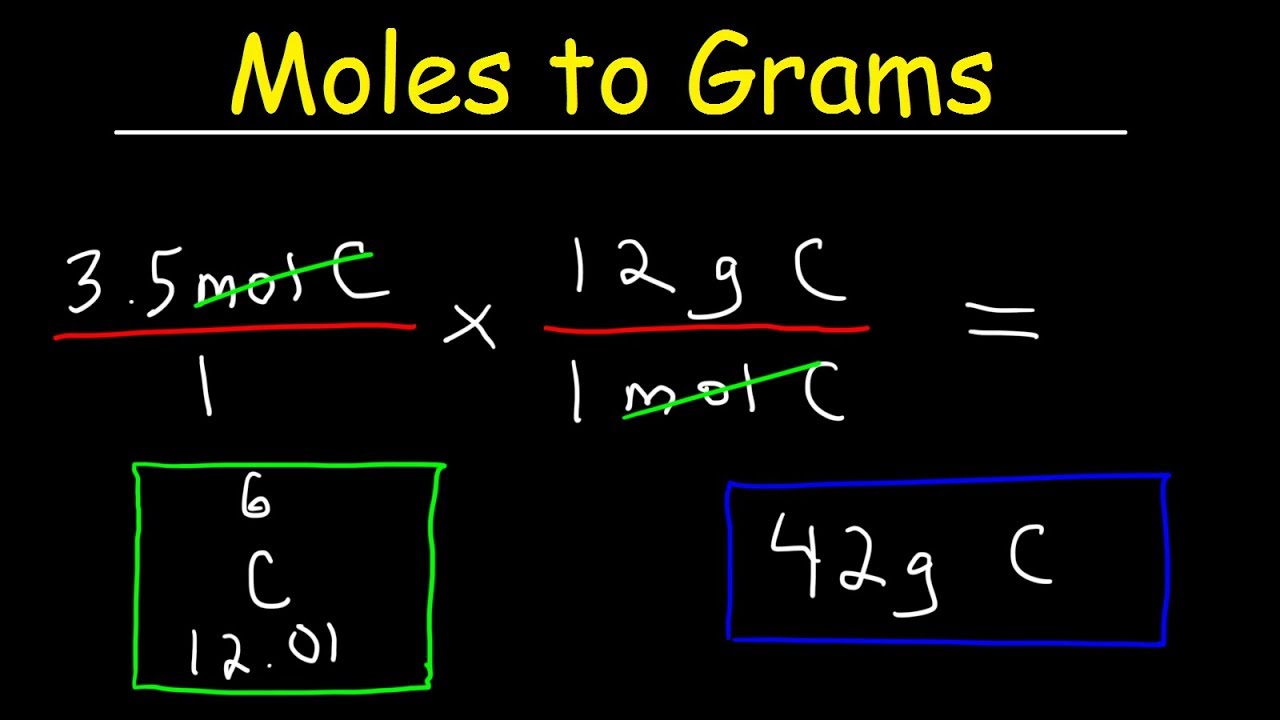
Formula to convert moles to grams.
To convert moles to g, we multiply the moles by the molar mass also known as the molecular weight.
Grams = Moles x Molar Mass
Example:
Convert 0.2 moles of Sodium chloride.
Since we know the molar mass of NaCl is 58.44g/mol, therefore we will multiply it by 0.2 to get grams.
= 0.2 x 58.44
= 11.688 grams.
You have three steps to convert mole values to grams.
- Calculate how many moles are mentioned in the question.
- Find the molar mass of the substance.
- Multiply both the values.
One mole consists of Avogadro number of atoms. If you know the quantity of mole, it can be converted into grams and vice versa.
The formula for moles to grams is given by
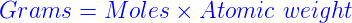
Example 1 –Calculate the mass in grams of 3.6 mol of H2SO4.
Solution
Look for the atomic masses of hydrogen, sulfur and oxygen.
H = 1.008
S = 32.06
O = 16
Therefore, the molecular mass of H2SO4 is
2(1.008) + 32.06 + 4(18) = 106.076
Hence, one mole of H2SO4 weights 106.076 grams.
Since you need to find for 3.60 mol of H2SO4
= 3.60 mol x 106.076 g/mol
= 381.87 g of H2SO4
Example 2.
Convert 3 moles of carbon monoxide to grams.
Solution
Find the molecular weight of Carbon monoxide.
By calculating, we get the molecular weight of CO = 28.01 g/mol
Since we need to find for 3 moles of Carbon monoxide
= 3 x 28.01 = 84.03 grams
Conclusion
People who have an understanding about the term conversion factor usally know that there are all types of factors that play a role in it. The factors include the value of the amount being converted, its country of originn and where it is being converted to.